Aspirin
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| 2-acetoxybenzoic acid | |
| Identifiers | |
| CAS number | 50-78-2 |
| ATC code | A01AD05 B01AC06, N02BA01 |
| PubChem | CID 2244 |
| DrugBank | DB00945 |
| ChemSpider | 2157 |
| Chemical data | |
| Formula | C9H8O4 |
| Mol. mass | 180.157 g/mol |
| SMILES | eMolecules & PubChem |
| Synonyms | 2-acetyloxybenzoic acid acetylsalicylate acetylsalicylic acid O-acetylsalicylic acid |
| Physical data | |
| Density | 1.40 g/cm³ |
| Melt. point | 135 °C (275 °F) |
| Boiling point | 140 °C (284 °F) (decomposes) |
| Solubility in water | 3 mg/mL (20 °C) |
| Pharmacokinetic data | |
| Bioavailability | Rapidly and completely absorbed |
| Protein binding | 99.6% |
| Metabolism | Hepatic |
| Half-life | 300–650 mg dose: 3.1–3.2 h 1 g dose: 5 h 2 g dose: 9 h |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | C(AU) D(US) |
| Legal status | Unscheduled (AU) GSL (UK) OTC (US) |
| Routes | Most commonly oral, also rectal. Lysine acetylsalicylate may be given IV or IM |
| |
|
Aspirin (USAN), also known as acetylsalicylic acid (pronounced /əˌsɛtəlˌsælɨˈsɪlɨk/ ə-SET-əl-sal-i-SIL-ik, abbreviated ASA), is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication.
Aspirin also has an antiplatelet effect by inhibiting the production of thromboxane, which under normal circumstances binds platelet molecules together to create a patch over damage of the walls within blood vessels. Because the platelet patch can become too large and also block blood flow, locally and downstream, aspirin is also used long-term, at low doses, to help prevent heart attacks, strokes, and blood clot formation in people at high risk for developing blood clots.[1] It has also been established that low doses of aspirin may be given immediately after a heart attack to reduce the risk of another heart attack or of the death of cardiac tissue.[2][3]
The main undesirable side effects of aspirin are gastrointestinal ulcers, stomach bleeding, and tinnitus, especially in higher doses. In children and adolescents, aspirin is no longer used to control flu-like symptoms or the symptoms of chickenpox or other viral illnesses, because of the risk of Reye's syndrome.[4]
Aspirin was the first discovered member of the class of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs), not all of which are salicylates, although they all have similar effects and most have inhibition of the enzyme cyclooxygenase as their mechanism of action. Today, aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year.[5] In countries where Aspirin is a registered trademark owned by Bayer, the generic term is acetylsalicylic acid (ASA).[6][7]
Contents |
History
A French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid in 1853. In the course of his work on the synthesis and properties of various acid anhydrides, he mixed acetyl chloride with a sodium salt of salicylic acid (sodium salicylate). A vigorous reaction ensued, and the resulting melt soon solidified.[8] Since no structural theory existed at that time, Gerhardt called the compound he obtained "salicylic-acetic anhydride" (wasserfreie Salicylsäure-Essigsäure). This preparation of aspirin ("salicylic-acetic anhydride") was one of the many reactions Gerhardt conducted for his paper on anhydrides and he did not pursue it further.

Six years later, in 1859, von Gilm obtained analytically pure acetylsalicylic acid (which he called "acetylierte Salicylsäure", acetylated salicylic acid) by a reaction of salicylic acid and acetyl chloride.[9] In 1869 Schröder, Prinzhorn and Kraut repeated both Gerhardt's (from sodium salicylate) and von Gilm's (from salicylic acid) syntheses and concluded that both reactions gave the same compound—acetylsalicylic acid. They were first to assign to it the correct structure with the acetyl group connected to the phenolic oxygen.[10]
In 1897, scientists at the drug and dye firm Bayer began investigating acetylsalicylic acid as a less-irritating replacement for standard common salicylate medicines. By 1899, Bayer had dubbed this drug Aspirin and was selling it around the world.[11] The name aspirin is derived from acetyl and "spirsäure" = an old (German) name for salicylic acid.[12] The popularity of aspirin grew over the first half of the twentieth century, spurred by its supposed effectiveness in the wake of the Spanish flu pandemic of 1918. However recent research suggests that the high death toll of the 1918 flu was partly due to aspirin, as the aspirin doses used at times can lead to toxicity, fluid in the lungs, and in some cases contribute to secondary bacterial infections and mortality.[13] Aspirin's profitability led to fierce competition and the proliferation of aspirin brands and products, especially after the American patent held by Bayer expired in 1917.[14][15]
The popularity of aspirin declined after the market releases of paracetamol (acetaminophen) in 1956 and ibuprofen in 1969.[16] In the 1960s and 1970s, John Vane and others discovered the basic mechanism of aspirin's effects, while clinical trials and other studies from the 1960s to the 1980s established aspirin's efficacy as an anti-clotting agent that reduces the risk of clotting diseases.[17] Aspirin sales revived considerably in the last decades of the twentieth century, and remain strong in the twenty-first century, because of its widespread use as a preventive treatment for heart attacks and strokes.[18]
Trademark in most countries
As part of war reparations specified in the 1919 Treaty of Versailles following Germany's surrender after World War I, Aspirin (along with heroin) lost its status as a registered trademark in France, Russia, the United Kingdom, and the United States, where it became a generic name and can be spelled in lower case.[19][20][21] Today, "aspirin" is a generic word in Australia, France, India, Ireland, New Zealand, Pakistan, the Philippines, South Africa, United Kingdom and the United States.[22] Aspirin, with a capital "A", remains a registered trademark of Bayer in Germany, Canada, Mexico, and in over 80 other countries, where the trademark is owned by Bayer, using a uniform chemical formula for all markets, but adapting the packaging and physical aspects for each.[23][24]
Therapeutic uses
Headache
Aspirin is one of the first-line drugs used in the treatment of migraine, bringing relief in 50–60% of the cases.[25]

It is as effective as a newer triptan medication sumatriptan (Imitrex)[26] and other painkillers such as paracetamol (acetaminophen)[27] or ibuprofen.[28] The combination of aspirin, paracetamol (acetaminophen) and caffeine (Excedrin) is even more potent. For the treatment of migraine headache, this formulation works better than any of its three components taken separately,[27] better than ibuprofen[29] and better than sumatriptan. Similarly to all other medications for migraine, it is recommended to take aspirin at the first signs of the headache, and it is the way these medications were used in the comparative clinical trials.[30]
Aspirin alleviates pain in 60-75% of patients with episodic tension headaches.[31][32] It is equivalent to paracetamol (acetaminophen) in that respect, except for the higher frequency of gastrointestinal side effects.[32] Comparative clinical trials indicated that metamizole and ibuprofen may relieve pain faster than aspirin, although the difference becomes insignificant after about 2 hours. The addition of Caffeine in a dose of 60 – 130 mg to aspirin increases the analgesic effect in headache.[31][33] The combination of aspirin, paracetamol (acetaminophen) and caffeine (Excedrin) is still more effective, but at the cost of more stomach discomfort, nervousness and dizziness.[34]
Pain
In general, aspirin works well for dull, throbbing pain; it is ineffective for pain caused by most muscle cramps, bloating, gastric distension and acute skin irritation.[35] The most studied example is pain after surgery such as tooth extraction, for which the highest allowed dose of aspirin (1 g) is equivalent to 1 g of paracetamol (acetaminophen), 60 mg of codeine and 5 mg of oxycodone. Combination of aspirin and caffeine, generally, affords greater pain relief than aspirin alone. Effervescent aspirin alleviates pain much faster than aspirin in tablets (15–30 min vs. 45–60 min).[36]
Nevertheless, as a post-surgery painkiller, aspirin is inferior to ibuprofen. Aspirin has higher gastrointestinal toxicity than ibuprofen. The maximum dose of aspirin (1 g) provides weaker pain relief than an intermediate dose of ibuprofen (400 mg), and this relief does not last as long.[36] A combination of aspirin and codeine may have a slightly higher analgesic effect than aspirin alone; however, this difference is not clinically meaningful.[37] It appears that ibuprofen is at least equally, and possibly more, effective than this combination.[38]
According to a meta-analysis of clinical trials for menstrual pain, aspirin demonstrated higher efficacy than placebo but lower one than ibuprofen or naproxen, although maximum doses of aspirin were never used in these trials. The authors concluded that ibuprofen has the best risk-benefit ratio.[39]
Aspirin did not ease pain during cycling exercise,[40] while caffeine, surprisingly, was very effective.[41][42] Similarly, aspirin, codeine or paracetamol (acetaminophen) were not better than placebo for muscle soreness after exercise.[43]
Prevention of heart attacks and strokes
There are two distinct uses of aspirin for prophylaxis of cardiovascular events: primary prevention and secondary prevention. Primary prevention is about decreasing strokes and heart attacks in the general population of those who have no diagnosed heart or vascular problems. Secondary prevention concerns patients with known cardiovascular disease.[44]
Low doses of aspirin are recommended for the secondary prevention of strokes and heart attacks. For both males and females diagnosed with cardiovascular disease, aspirin reduces the chance of a heart attack and ischaemic stroke by about a fifth. This translates to an absolute rate reduction from 8.2% to 6.7% of such events per year for people already with cardiovascular disease. Although aspirin also raises the risk of hemorrhagic stroke and other major bleeds by about twofold, these events are rare, and the balance of aspirin's effects is positive. Thus, in secondary prevention trials, aspirin reduced the overall mortality by about a tenth.[44]
For persons without cardiovascular problems, the benefits of aspirin are unclear. In the primary prevention trials aspirin decreased the overall incidence of heart attacks and ischaemic strokes by about a tenth. However, since these events were rare, the absolute reduction of their rate was low: from 0.57% to 0.51% per year. In addition, the risks of hemorrhagic strokes and gastrointestinal bleeding almost completely offset the benefits of aspirin. Thus, in the primary prevention trials aspirin did not change the overall mortality rate.[44] There is continuing discussion and debate in the scientific community regarding these topics.[45]
The expert bodies diverge in their opinions regarding the use of aspirin for primary prevention. The US Government Preventive Services Task Force recommended making individual case by case choice based on the estimated future risk and patient's preferences.[46][47] On the other hand, Antithrombotic Trialists’ Collaboration argued that such recommendations are unjustified since the relative reduction of risk in the primary prevention trials of aspirin was same for persons in high- and low-risk groups and did not depend on the blood pressure. The Collaboration suggested statins as the alternative and more effective preventive medication.[44]
Coronary and carotid arteries, bypasses and stents
The coronary arteries supply blood to the heart. Aspirin is recommended for 1 to 6 months after placement of stents in the coronary arteries and for years after a coronary artery bypass graft.
The carotid arteries supply blood to the brain. Patients with mild carotid artery stenosis benefit from aspirin. Aspirin is recommended after a carotid endarterectomy or carotid artery stent.
After vascular surgery of the lower legs using artificial grafts which are sutured to the arteries to improve blood supply, aspirin is used to keep the grafts open.
Other uses
Although aspirin has been used to combat fever and pains associated with common cold for more than 100 years, only recently its efficacy was confirmed in controlled clinical trials on adults. 1 g of aspirin, on average, reduced the oral body temperature from 39.0 °C (102.2 °F) to 37.6 °C (99.7 °F) after 3 hours. The relief began after 30 minutes, and after 6 hours the temperature still remained below 37.8 °C (100.0 °F). Aspirin also helped with "achiness", discomfort and headache,[48] and with sore throat pain, for those who had it.[49] Aspirin was indistinguishable from paracetamol (acetaminophen) in any respect, except for, possibly, slightly higher rate of sweating and gastrointestinal side effects.[48]
Fever and joint pain of acute rheumatic fever respond extremely well, often within three days, to high doses of aspirin. The therapy usually lasts for 1–2 weeks; and only in about 5% of the cases it has to continue for longer than six months. After fever and pain have subsided, the aspirin treatment is unnecessary as it does not decrease the incidence of heart complications and residual rheumatic heart disease.[50] In addition, the high doses of aspirin used cause liver toxicity in about 20% of the treated children,[51][52] who are the majority of rheumatic fever patients, and increase the risk of them developing Reye's syndrome.[50] Naproxen was shown to be as effective as aspirin and less toxic; however, due to the limited clinical experience, naproxen is recommended only as a second-line treatment.[50][53]
Along with rheumatic fever, Kawasaki disease remains one of the few indications for aspirin use in children, although even this use has been questioned by some authors.[54] In the United Kingdom, the only indications for aspirin use in children and adolescents under 16 are Kawasaki disease and prevention of blood clot formation.
Aspirin is also used in the treatment of pericarditis, coronary artery disease, and acute myocardial infarction.[55][56][57]
Experimental
Aspirin has been theorized to reduce cataract formation in diabetic patients, but one study showed it was ineffective for this purpose.[58] The role of aspirin in reducing the incidence of many forms of cancer has also been widely studied. In several studies, aspirin use did not reduce the incidence of prostate cancer.[59][60] Its effects on the incidence of pancreatic cancer are mixed; one study published in 2004 found a statistically significant increase in the risk of pancreatic cancer among women,[61] while a meta-analysis of several studies, published in 2006, found no evidence that aspirin or other NSAIDs are associated with an increased risk for the disease.[62] The drug may be effective in reduction of risk of various cancers, including those of the colon,[63][64][65][66] lung,[67][68] and possibly the upper GI tract, though some evidence of its effectiveness in preventing cancer of the upper GI tract has been inconclusive.[69][69][70] Its preventative effect against adenocarcinomas may be explained by its inhibition of PTGS2 (COX-2) enzymes expressed in them.[71]
In a 2009 article published by the Journal of Clinical Investigation, it was found that aspirin might prevent liver damage. In their experiment, scientists from Yale University and The University of Iowa induced damage in certain liver cells called hepatocytes using excessive doses of acetaminophen. This caused hepatoxicity and hepatocyte death which triggered an increase in the production of TLR9. The expression of TLR9 caused an inflammatory cascade involving pro–IL-1β and pro-IL-18. Aspirin was found to have a protective effect on hepatocytes because it led to the "downregulation of proinflammatory cytokines".[72]
In another 2009 article published by the Journal of the American Medical Association, it was found that men and women who regularly took aspirin after colorectal cancer diagnosis had lower risk of overall and colorectal cancer death compared to patients not using aspirin.[73][74]
A 2010 article in the Journal of Clinical Oncology has suggested that aspirin may reduce the risk of death from breast cancer[75]. While the information has been well-circulated by the media[76][77], official health bodies and medical groups have expressed concern over the touting of aspirin as "miracle drug"[78].
Contraindications and resistance
Aspirin should not be taken by people who are allergic to ibuprofen or naproxen,[79][80] or who have salicylate intolerance[81][82] or a more generalized drug intolerance to NSAIDs, and caution should be exercised in those with asthma or NSAID-precipitated bronchospasm. Owing to its effect on the stomach lining, manufacturers recommend that people with peptic ulcers, mild diabetes, or gastritis seek medical advice before using aspirin.[79][83] Even if none of these conditions are present, there is still an increased risk of stomach bleeding when aspirin is taken with alcohol or warfarin.[79][80] Patients with hemophilia or other bleeding tendencies should not take aspirin or other salicylates.[79][83] Aspirin is known to cause hemolytic anemia in people who have the genetic disease glucose-6-phosphate dehydrogenase deficiency (G6PD), particularly in large doses and depending on the severity of the disease.[84][85] Use of aspirin during dengue fever is not recommended owing to increased bleeding tendency.[86] People with kidney disease, hyperuricemia, or gout should not take aspirin because aspirin inhibits the kidneys' ability to excrete uric acid and thus may exacerbate these conditions. Aspirin should not be given to children or adolescents to control cold or influenza symptoms as this has been linked with Reye's syndrome.[4]
For some people, aspirin does not have as strong an effect on platelets as for others, an effect known as aspirin resistance or insensitivity. One study has suggested that women are more likely to be resistant than men[87] and a different, aggregate study of 2,930 patients found 28% to be resistant.[88] A study in 100 Italian patients found that of the apparent 31% aspirin resistant subjects, only 5% were truly resistant, and the others were noncompliant.[89]
Adverse effects
Gastrointestinal
Aspirin use has been shown to increase the risk of gastrointestinal bleeding.[90] Although some enteric coated formulations of aspirin are advertised as being "gentle to the stomach", in one study enteric coating did not seem to reduce this risk.[90] Combining aspirin with other NSAIDs has also been shown to further increase this risk.[90] Using aspirin in combination with clopidogrel or warfarin also increases the risk of upper gastrointestinal bleeding.[91]
Central effects
Large doses of salicylate, a metabolite of aspirin, have been proposed to cause tinnitus (ringing in the ears) based on the experiments in rats, via the action on arachidonic acid and NMDA receptors cascade.[92]
Reye's syndrome
Reye's syndrome, a severe illness characterized by acute encephalopathy and fatty liver, can occur when children or adolescents are given aspirin for a fever or other illnesses or infections. From 1981 through 1997, 1207 cases of Reye's syndrome in under-18 patients were reported to the U.S. Centers for Disease Control and Prevention. Of these, 93% reported being ill in the three weeks preceding onset of Reye's syndrome, most commonly with a respiratory infection, chickenpox, or diarrhea. Salicylates were detectable in 81.9% of children for whom test results were reported.[93] After the association between Reye's syndrome and aspirin was reported and safety measures to prevent it (including a Surgeon General's warning and changes to the labeling of aspirin-containing drugs) were implemented, aspirin-taking by children declined considerably in the United States, as did the number of reported cases of Reye's syndrome; a similar decline was found in the United Kingdom after warnings against pediatric aspirin use were issued.[93] The United States Food and Drug Administration now recommends that aspirin (or aspirin-containing products) should not be given to anyone under the age of 12 who has a fever,[4] and the British Medicines and Healthcare products Regulatory Agency (MHRA) recommends that children who are under 16 years of age should not take aspirin, unless it is on the advice of a doctor.[94]
Hives/swelling
For a small number of people, aspirin can result in symptoms that resemble an allergic reaction and include hives, swelling, and headache. The reaction is caused by salicylate intolerance and is not a true allergy but rather an inability to metabolize even small amounts of aspirin, resulting in an overdose.
Other effects
Aspirin can induce angioedema in some people. In one study, angioedema appeared 1–6 hours after ingesting aspirin in some of the patients participating in the study. However, when the aspirin was taken alone it did not cause angioedema in these patients; the aspirin had been taken in combination with another NSAID-induced drug when angioedema appeared.[95]
Aspirin causes an increased risk of cerebral microbleeds that is the appearance on MRI scans of 5–10 mm or smaller hypointense (dark holes) patches.[96][97] Such cerebral microbleeds are important since they often occur prior to ischemic stroke or intracerebral hemorrhage, Binswanger disease and Alzheimers Disease.
Aspirin can cause prolonged bleeding after operations for up to 10 days. In one study, thirty patients were observed after their various surgeries. Twenty of the thirty patients had to have an additional unplanned operation because of postoperative bleeding.[98] This diffuse bleeding was associated with aspirin alone or in combination with another NSAID in 19 out of the 20 who had to have another operation owing to bleeding after their operation. The average recovery time for the second operation was 11 days.
| Condition | Prothrombin time | Partial thromboplastin time | Bleeding time | Platelet count |
|---|---|---|---|---|
| Vitamin K deficiency or warfarin | prolonged | prolonged | unaffected | unaffected |
| Disseminated intravascular coagulation | prolonged | prolonged | prolonged | decreased |
| Von Willebrand disease | unaffected | prolonged | prolonged | unaffected |
| Haemophilia | unaffected | prolonged | unaffected | unaffected |
| Aspirin | unaffected | unaffected | prolonged | unaffected |
| Thrombocytopenia | unaffected | unaffected | prolonged | decreased |
| Early Liver failure | prolonged | unaffected | unaffected | unaffected |
| End-stage Liver failure | prolonged | prolonged | prolonged | decreased |
| Uremia | unaffected | unaffected | prolonged | unaffected |
| Congenital afibrinogenemia | prolonged | prolonged | prolonged | unaffected |
| Factor V deficiency | prolonged | prolonged | unaffected | unaffected |
| Factor X deficiency as seen in amyloid purpura | prolonged | prolonged | unaffected | unaffected |
| Glanzmann's thrombasthenia | unaffected | unaffected | prolonged | unaffected |
| Bernard-Soulier syndrome | unaffected | unaffected | prolonged | decreased |
Dosage

For adults doses are generally taken four times a day for fever or arthritis,[99] with doses near the maximal daily dose used historically for the treatment of rheumatic fever.[100] For the prevention of myocardial infarction in someone with documented or suspected coronary artery disease, much lower doses are taken once daily.[99]
New recommendations from the US Preventive Services Task Force (USPSTF, March, 2009) on the use of aspirin for the primary prevention of coronary heart disease encourage men aged 45–79 and women aged 55–79 to use aspirin when the potential benefit of a reduction in myocardial infarction (MI) for men or stroke for women outweighs the potential harm of an increase in gastrointestinal hemorrhage. Regular low dose (75 to 81 mg) aspirin users had a 25% lower risk of death from cardiovascular disease and a 14% lower risk of death from any cause. Low dose aspirin use was also associated with a trend toward lower risk of cardiovascular events, and lower aspirin doses (75 to 81 mg/day) may optimize efficacy and safety for patients requiring aspirin for long-term prevention.[101]
In children with Kawasaki disease, aspirin is taken at dosages based on body weight, initially four times a day for up to two weeks and then at a lower dose once daily for a further six to eight weeks.[102]
Overdose
Aspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, higher than normal doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal with a mortality rate of 25%; chronic overdose may be especially severe in children.[103] Toxicity is managed with a number of potential treatments including: activated charcoal, intravenous dextrose and normal saline, sodium bicarbonate, and dialysis.[104]
Mechanism of action
Discovery of the mechanism
In 1971, British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons in London, showed that aspirin suppressed the production of prostaglandins and thromboxanes.[105][106] For this discovery, he was awarded both a Nobel Prize in Physiology or Medicine in 1982 and a knighthood.
Suppression of prostaglandins and thromboxanes
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (PTGS) enzyme. Cyclooxygenase is required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.
Low-dose, long-term aspirin use irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation. This anticoagulant property makes aspirin useful for reducing the incidence of heart attacks.[107] 40 mg of aspirin a day is able to inhibit a large proportion of maximum thromboxane A2 release provoked acutely, with the prostaglandin I2 synthesis being little affected; however, higher doses of aspirin are required to attain further inhibition.[108]
Prostaglandins are local hormones produced in the body and have diverse effects in the body, including the transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation. Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are primarily caused by blood clots, and low doses of aspirin are seen as an effective medical intervention for acute myocardial infarction. The major side effect of this is that because the ability of blood to clot is reduced, excessive bleeding may result from the use of aspirin.
PTGS1 [COX-1] and PTGS2 [COX-2] inhibition
There are at least two different types of cyclooxygenase: PTGS1 and PTGS2. Aspirin irreversibly inhibits PTGS1 and modifies the enzymatic activity of PTGS2. Normally PTGS2 produces prostanoids, most of which are pro-inflammatory. Aspirin-modified PTGS2 produces lipoxins, most of which are anti-inflammatory. Newer NSAID drugs called PTGS2 selective inhibitors have been developed that inhibit only PTGS2, with the intent to reduce the incidence of gastrointestinal side effects.[5]
However, several of the new PTGS2 selective inhibitors, such as Vioxx, have been withdrawn recently, after evidence emerged that PTGS2 inhibitors increase the risk of heart attack. It is proposed that endothelial cells lining the microvasculature in the body express PTGS2, and, by selectively inhibiting PTGS2, prostaglandin production (specifically PGI2; prostacyclin) is downregulated with respect to thromboxane levels, as PTGS1 in platelets is unaffected. Thus, the protective anti-coagulative effect of PGI2 is removed, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new PTGS once aspirin has irreversibly inhibited the enzyme, an important difference with reversible inhibitors.
Additional mechanisms
Aspirin has been shown to have at least three additional modes of action. It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, by diffusing from the inner membrane space as a proton carrier back into the mitochondrial matrix, where it ionizes once again to release protons.[109] In short, aspirin buffers and transports the protons. When high doses of aspirin are given, aspirin may actually cause fever owing to the heat released from the electron transport chain, as opposed to the antipyretic action of aspirin seen with lower doses. Additionally, aspirin induces the formation of NO-radicals in the body, which have been shown in mice to have an independent mechanism of reducing inflammation. This reduced leukocyte adhesion, which is an important step in immune response to infection; however, there is currently insufficient evidence to show that aspirin helps to fight infection.[110] More recent data also suggests that salicylic acid and its derivatives modulate signaling through NF-κB.[111] NF-κB is a transcription factor complex that plays a central role in many biological processes, including inflammation.
Effects upon Hypothalamic-Pituitary-Adrenal Activity
Aspirin reduces the effects of vasopressin[112] and increases those of naloxone[113] upon the secretion of ACTH and cortisol by the hypothalamic-pituitary-adrenal axis. It has been suggested that this occurs through an interaction with endogenous prostaglandins and their role in regulating the HPA axis.[112]
Pharmacokinetics
Salicylic acid is a weak acid, and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, which can delay absorption of high doses for 8 to 24 hours. In addition to the increased pH of the small intestine, aspirin is rapidly absorbed there owing to the increased surface area, which in turn allows more of the salicylate to dissolve. Owing to the issue of solubility, however, aspirin is absorbed much more slowly during overdose, and plasma concentrations can continue to rise for up to 24 hours after ingestion.[114][115][116]
About 50–80% of salicylate in the blood is bound by protein while the rest remains in the active, ionized state; protein binding is concentration-dependent. Saturation of binding sites leads to more free salicylate and increased toxicity. The volume of distribution is 0.1–0.2 l/kg. Acidosis increases the volume of distribution because of enhancement of tissue penetration of salicylates.[116]
As much as 80% of therapeutic doses of salicylic acid is metabolized in the liver. Conjugation with glycine forms salicyluric acid and with glucuronic acid forms salicyl acyl and phenolic glucuronide. These metabolic pathways have only a limited capacity. Small amounts of salicylic acid are also hydroxylated to gentisic acid. With large salicylate doses, the kinetics switch from first order to zero order, as metabolic pathways become saturated and renal excretion becomes increasingly important.[116]
Salicylates are excreted mainly by the kidneys as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%) and acyl (5%) glucuronides, and gentisic acid (< 1%). When small doses (less than 250 mg in an adult) are ingested, all pathways proceed by first-order kinetics, with an elimination half-life of about 2 to 4.5 hours.[117][118] When higher doses of salicylate are ingested (more than 4 g), the half-life becomes much longer (15–30 hours)[119] because the biotransformation pathways concerned with the formation of salicyluric acid and salicyl phenolic glucuronide become saturated.[120] Renal excretion of salicylic acid becomes increasingly important as the metabolic pathways become saturated, because it is extremely sensitive to changes in urinary pH. There is a 10 to 20 fold increase in renal clearance when urine pH is increased from 5 to 8. The use of urinary alkalinization exploits this particular aspect of salicylate elimination.[121]
Interactions
Aspirin is known to interact with other drugs. For example, acetazolamide and ammonium chloride have been known to enhance the intoxicating effect of salicyclates, and alcohol also increases the gastrointestinal bleeding associated with these types of drugs.[79][80] Aspirin is known to displace a number of drugs from protein binding sites in the blood, including the anti-diabetic drugs tolbutamide and chlorpropamide, the immunosuppressant methotrexate, phenytoin, probenecid, valproic acid (as well as interfering with beta oxidation, an important part of valproate metabolism) and any nonsteroidal anti-inflammatory drug. Corticosteroids may also reduce the concentration of aspirin. The pharmacological activity of spironolactone may be reduced by taking aspirin, and aspirin is known to compete with Penicillin G for renal tubular secretion.[122] Aspirin may also inhibit the absorption of vitamin C.[123][124][125]
Veterinary uses
Aspirin has been used to treat pain and arthritis in veterinary medicine, primarily in dogs, although it is often not recommended for this purpose, as there are newer medications available with fewer side effects in these animals. Dogs, for example, are particularly susceptible to the gastrointestinal side effects associated with salicylates.[126] Horses have also been given aspirin for pain relief, although it is not commonly used owing to its relatively short-lived analgesic effects. Horses are also fairly sensitive to the gastrointestinal side effects. Nevertheless, it has shown promise in its use as an anticoagulant, mostly in cases of laminitis.[127] Aspirin should only be used in animals under the direct supervision of a veterinarian. Aspirin should never be given to cats because they lack the ability to form glucuronide conjugates, which makes it more likely that aspirin will be toxic. Toxicity may be reduced by administering dosages at longer intervals.[128]
Chemistry
Aspirin is an acetyl derivative of salicylic acid that is a white, crystalline, weakly acidic substance, with a melting point of 135 °C (275 °F). Acetylsalicylic acid decomposes rapidly in solutions of ammonium acetate or of the acetates, carbonates, citrates or hydroxides of the alkali metals. Acetylsalicylic acid is stable in dry air, but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis, the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate.[129]
Synthesis
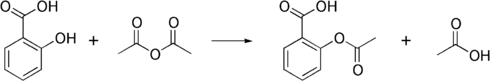
The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid's hydroxyl group into an acetyl group, (R-OH → R-OCOCH3). This process yields aspirin and acetic acid, which is considered a byproduct of this reaction. Small amounts of sulfuric acid (and occasionally phosphoric acid) are almost always used as a catalyst. This method is commonly employed in undergraduate teaching labs.[130]
Formulations containing high concentrations of aspirin often smell like vinegar.[131] This is because aspirin can decompose through hydrolysis in moist conditions, yielding salicylic acid and acetic acid.[132]
The acid dissociation constant (pKa) for acetylsalicylic acid is 3.5 at 25 °C (77 °F).[133]
Polymorphism
Polymorphism, or the ability of a substance to form more than one crystal structure, is important in the development of pharmaceutical ingredients. Many drugs are receiving regulatory approval for only a single crystal form or polymorph. For a long time, only one crystal structure for aspirin was known, although there had been indications that aspirin might have a second crystalline form since the 1960s. The elusive second polymorph was first discovered by Vishweshwar and coworkers in 2005,[134] and fine structural details were given by Bond et al.[135] A new crystal type was found after attempted co-crystallization of aspirin and levetiracetam from hot acetonitrile. The form II is only stable at 100 K and reverts back to form I at ambient temperature. In the (unambiguous) form I, two salicylic molecules form centrosymmetric dimers through the acetyl groups with the (acidic) methyl proton to carbonyl hydrogen bonds, and in the newly claimed form II, each salicylic molecule forms the same hydrogen bonds with two neighboring molecules instead of one. With respect to the hydrogen bonds formed by the carboxylic acid groups both polymorphs form identical dimer structures.
Compendial status
See also
- Aspergum
- blood platelets
- copper aspirinate
- nonsteroidal anti-inflammatory drug
- history of aspirin
- salicylic acid
- thrombocytopenic purpura
- ibuprofen
- paracetamol (acetaminophen)
- naproxen
- Choline Magnesium Trisalicylate (Trilisate)
Notes and references
- ↑ Lewis, H D; J W Davis, D G Archibald, W E Steinke, T C Smitherman, J E Doherty, H W Schnaper, M M LeWinter, E Linares, J M Pouget, S C Sabharwal, E Chesler, H DeMots (1983-08-18). "Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study". The New England journal of medicine 309 (7): 396–403. doi:10.1056/NEJM198308183090703. ISSN 00284793. PMID 6135989.
- ↑ Julian, D G; D A Chamberlain, S J Pocock (1996-09-24). "A comparison of aspirin and anticoagulation following thrombolysis for myocardial infarction (the AFTER study): a multicentre unblinded randomised clinical trial". BMJ (British Medical Journal) 313 (7070): 1429–1431. PMID 8973228.
- ↑ Krumholz, Harlan M.; Martha J. Radford, Edward F. Ellerbeck, John Hennen, Thomas P. Meehan, Marcia Petrillo, Yun Wang, Timothy F. Kresowik, Stephen F. Jencks (1995). "Aspirin in the Treatment of Acute Myocardial Infarction in Elderly Medicare Beneficiaries : Patterns of Use and Outcomes". Circulation 92 (10): 2841–2847. PMID 7586250.
- ↑ 4.0 4.1 4.2 Macdonald S (2002). "Aspirin use to be banned in under 16 year olds". BMJ 325 (7371): 988. doi:10.1136/bmj.325.7371.988/c. PMID 12411346.
- ↑ 5.0 5.1 Warner, T. D.; Warner TD, Mitchell JA. (2002). "Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?". Proc Natl Acad Sci USA 99 (21): 13371–3. doi:10.1073/pnas.222543099. PMID 12374850. PMC 129677. http://www.pnas.org/cgi/content/extract/99/21/13371.
- ↑ http://www.wordconstructions.com/articles/health/aspirin.html
- ↑ http://www.inta.org/index.php?option=com_content&task=view&id=202&Itemid=126&getcontent=5
- ↑ (German) Gerhardt C (1853). "Untersuchungen über die wasserfreien organischen Säuren". Annalen der Chemie und Pharmacie 87: 149–179. doi:10.1002/jlac.18530870107.
- ↑ (German) von Gilm H (1859). "Acetylderivate der Phloretin- und Salicylsäure". Annalen der Chemie und Pharmacie 112 (2): 180–185. doi:10.1002/jlac.18591120207.
- ↑ (German) Schröder, Prinzhorn, Kraut K (1869). "Uber Salicylverbindungen". Annalen der Chemie und Pharmacie 150 (1): 1–20. doi:10.1002/jlac.18691500102.
- ↑ Jeffreys, Diarmuid (August 11, 2005). Aspirin: The Remarkable Story of a Wonder Drug. Bloomsbury USA. p. 73. ISBN 1582346003.
- ↑ Ueber Aspirin. Pflügers Archiv : European journal of physiology, Volume: 84, Issue: 11-12 (March 1, 1901), pp: 527-546.
- ↑ Karen M. Starko. Salicylates and Pandemic Influenza Mortality, 1918%u20131919 Pharmacology, Pathology, and Historic Evidence. Clinical Infectious Diseases, 2009; DOI: 10.1086/606060
- ↑ Jeffreys, Aspirin, pp. 136–142 and 151-152
- ↑ http://www.history.com/this-day-in-history.do?action=VideoArticle&id=52415
- ↑ Jeffreys, Aspirin, pp. 212–217
- ↑ Jeffreys, Aspirin, pp. 226–231
- ↑ Jeffreys, Aspirin, pp. 267–269
- ↑ "Treaty of Versailles, Part X, Section IV, Article 298". 1919-06-28. pp. Annex, Paragraph 5. http://en.wikisource.org/wiki/Treaty_of_Versailles/Part_X#Article_298. Retrieved 2008-10-25.
- ↑ Mehta, Aalok (2005). "Aspirin". Chemical & Engineering News 83 (25). http://pubs.acs.org/cen/coverstory/83/8325/8325aspirin.html. Retrieved 2008-10-23.
- ↑ http://www.ul.ie/~childsp/CinA/Issue59/TOC43_Aspirin.htm
- ↑ CBE Style Manual Committee; Huth, Edward J. (1994). Scientific Style and Format: The CBE Manual for Authors, Editors, and Publishers. Cambridge University Press. p. 164. ISBN 9780521471541. http://books.google.com/books?id=PoFJ-OhE63UC&pg=PA164.
- ↑ "Aspirin: the versatile drug". CBC News. 2009-05-28. http://www.cbc.ca/health/story/2009/05/28/f-aspirin-studies.html.
- ↑ Cheng, Tsung O. (2007). "The History of Aspirin". Texas Heart Institute Journal 34 (3): 392–393. PMID 17948100.
- ↑ Tfelt-Hansen P (2008). "Triptans vs Other Drugs for Acute Migraine. Are There Differences in Efficacy? A Comment". Headache 48 (4): 601–605. doi:10.1111/j.1526-4610.2008.01064.x. PMID 18377382.
- ↑ Lampl C, Voelker M, Diener HC (2007). "Efficacy and safety of 1,000 mg effervescent aspirin: individual patient data meta-analysis of three trials in migraine headache and migraine accompanying symptoms". J Neurol 254 (6): 705–712. doi:10.1007/s00415-007-0547-2. PMID 17406776.
- ↑ 27.0 27.1 Diener HC, Bussone G, de Liano H et al (2004). "The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study". Cephalalgia 25 (10): 776–787. doi:10.1111/j.1468-2982.2005.00948.x. PMID 16162254.
- ↑ Diener HC, Pfaffenrath V, Pageler L et al (2004). "Efficacy and safety of 1,000 mg effervescent aspirin: individual patient data meta-analysis of three trials in migraine headache and migraine accompanying symptoms". Cephalalgia 24 (11): 947–54. doi:10.1111/j.1468-2982.2004.00783.x. PMID 15482357.
- ↑ Goldstein J, Silberstein SD, Saper JR et al (2006). "Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study". Headache 46 (3): 444–53. doi:10.1111/j.1526-4610.2006.00376.x. PMID 16618262.
- ↑ Goldstein J, Silberstein SD, Saper JR et al (2005). "Acetaminophen, aspirin, and caffeine versus sumatriptan succinate in the early treatment of migraine: results from the ASSET trial". Headache 45 (8): 973–82. doi:10.1111/j.1526-4610.2005.05177.x. PMID 16109110.
- ↑ 31.0 31.1 PMID 11472387 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 32.0 32.1 PMID 12534583 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 8706118 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 7955822 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 14592563 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 36.0 36.1 PMID 10868553 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 9373807 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 6763202 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 9692420 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 9268956 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 18458355 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 14622688 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 10896014 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 44.0 44.1 44.2 44.3 Baigent C, Blackwell L, Collins R, et al. (2009). "Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials". Lancet 373 (9678): 1849–60. doi:10.1016/S0140-6736(09)60503-1. PMID 19482214.
- ↑ The Danger of Daily Aspirin, By ANNA WILDE MATHEWS, Wall St Journal, FEBRUARY 23, 2010.
- ↑ Wolff T, Miller T, Ko S (2009). "Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force". Ann. Intern. Med. 150 (6): 405–10. PMID 19293073.
- ↑ US Preventive Services Task Force (2009). "Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement". Ann. Intern. Med. 150 (6): 396–404. PMID 19293072.
- ↑ 48.0 48.1 PMID 16154478 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 12873261 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 50.0 50.1 50.2 National Heart Foundation of Australia (RF/RHD guideline development working group) and the Cardiac Society of Australia and New Zealand (2006). "Diagnosis and management of acute rheumatic fever and rheumatic heart disease in Australia. An evidence-based review" (PDF). National Heart Foundation of Australia. pp. 33–37. http://www.heartfoundation.org.au/SiteCollectionDocuments/PP-590%20Diagnosis-Management%20ARF-RHD%20Evidence-Based%20Review.pdf. Retrieved 2009-11-01.
- ↑ PMID 14651540 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 1376585 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 14517527 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM (2004). "Treatment of acute Kawasaki disease: aspirin's role in the febrile stage revisited". Pediatrics 114 (6): e689–93. doi:10.1542/peds.2004-1037. PMID 15545617. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=15545617.
- ↑ Krumholz, HM; Radford MJ, Ellerbeck EF, Hennen J, Meehan TP, Petrillo M, Wang Y, Kresowik TF, Jencks SF. (1995). "Aspirin in the treatment of acute myocardial infarction in elderly Medicare beneficiaries. Patterns of use and outcomes". Circulation 92 (10): 2841–7. PMID 7586250.
- ↑ ISIS-2 Collaborative group (1988). "Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2". Lancet 2 (2): 349–60. PMID 2899772.
- ↑ Mallinson, T (2010). "Myocardial Infarction". Focus on First Aid (15): 15. http://www.focusonfirstaid.co.uk/Magazine/issue15/index.aspx. Retrieved 2010-06-08.
- ↑ Chew EY, Williams GA, Burton TC, Barton FB, Remaley NA, Ferris FL (1992). "Aspirin effects on the development of cataracts in patients with diabetes mellitus. Early treatment diabetic retinopathy study report 16". Arch Ophthalmol 110 (3): 339–42. PMID 1543449.
- ↑ Bosetti, et al.; Talamini, R; Negri, E; Franceschi, S; Montella, M; La Vecchia, C (2006). "Aspirin and the risk of prostate cancer". Eur J Cancer Prev 15 (1): 43–5. doi:10.1097/01.cej.0000180665.04335.de. PMID 16374228.
- ↑ Menezes, et al.; Swede, H; Niles, R; Moysich, KB (2006). "Regular use of aspirin and prostate cancer risk (United States)". Cancer Causes & Control 17 (3): 251–6. doi:10.1007/s10552-005-0450-z. PMID 16489532.
- ↑ Schernhammer, et al.; Kang, JH; Chan, AT; Michaud, DS; Skinner, HG; Giovannucci, E; Colditz, GA; Fuchs, CS (2004). "A Prospective Study of Aspirin Use and the Risk of Pancreatic Cancer in Women". J Natl Cancer Inst 96 (1): 22–28. doi:10.1093/jnci/djh001. PMID 14709735. http://jnci.oxfordjournals.org/cgi/content/full/96/1/22.
- ↑ Larsson SC, Giovannucci E, Bergkvist L, Wolk A (2006). "Aspirin and nonsteroidal anti-inflammatory drug use and risk of pancreatic cancer: a meta-analysis". Cancer Epidemiol. Biomarkers Prev. 15 (12): 2561–4. doi:10.1158/1055-9965.EPI-06-0574. PMID 17164387. http://cebp.aacrjournals.org/cgi/content/full/15/12/2561.
- ↑ Thun MJ, Namboodiri MM, Heath CW (1991). "Aspirin use and reduced risk of fatal colon cancer". N Engl J Med 325 (23): 1593–6. doi:10.1056/NEJM199112053252301. PMID 1669840.
- ↑ Baron, et al.; Cole, BF; Sandler, RS; Haile, RW; Ahnen, D; Bresalier, R; McKeown-Eyssen, G; Summers, RW et al. (2003). "A randomized trial of aspirin to prevent colorectal adenomas". N Engl J Med 348 (10): 891–9. doi:10.1056/NEJMoa021735. PMID 12621133.
- ↑ Chan, et al.; Giovannucci, EL; Schernhammer, ES; Colditz, GA; Hunter, DJ; Willett, WC; Fuchs, CS (2004). "A Prospective Study of Aspirin Use and the Risk for Colorectal Adenoma". Ann Intern Med 140 (3): 157–66. PMID 14757613.
- ↑ Chan, et al.; Giovannucci, EL; Meyerhardt, JA; Schernhammer, ES; Curhan, GC; Fuchs, CS (2005). "Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer". JAMA 294 (8): 914–23. doi:10.1001/jama.294.8.914. PMID 16118381.
- ↑ Akhmedkhanov, et al.; Toniolo, P; Zeleniuch-Jacquotte, A; Koenig, KL; Shore, RE (2002). "Aspirin and lung cancer in women". Br J cancer 87 (11): 1337–8. doi:10.1038/sj.bjc.6600370. PMID 12085255.
- ↑ Moysich KB, Menezes RJ, Ronsani A, et al. (2002). "Regular aspirin use and lung cancer risk". BMC Cancer 2: 31. doi:10.1186/1471-2407-2-31. PMID 12453317. Free full text
- ↑ 69.0 69.1 Jayaprakash, Vijayvel; Jayaprakash V, Menezes RJ, Javle MM, McCann SE, Baker JA, Reid ME, Natarajan N, Moysich KB. (2006). "Regular aspirin use and esophageal cancer risk". Int J Cancer 119 (1): 202–7. doi:10.1002/ijc.21814. PMID 16450404.
- ↑ Bosetti, et al.; Talamini, R; Franceschi, S; Negri, E; Garavello, W; La Vecchia, C (2003). "Aspirin use and cancers of the upper aerodigestive tract". Br J Cancer 88 (5): 672–74. doi:10.1038/sj.bjc.6600820. PMID 12618872.
- ↑ Wolff, et al.; Saukkonen, K; Anttila, S; Karjalainen, A; Vainio, H; Ristimäki, A (15 November 1998). "Expression of cyclooxygenase-2 in human lung carcinoma". Cancer Research 58 (22): 4997–5001. PMID 9823297. http://cancerres.aacrjournals.org/cgi/content/abstract/58/22/4997.
- ↑ Imaeda, Avlin B.; Watanabe, Azuma; Sohail, Muhammad A.; Mahmood, Shamail; Mohamadnejad, Mehdi; Sutterwala, Fayyaz S.; Flavell, Richard A.; Mehal, Wajahat Z. (2009). "Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome". Journal of Clinical Investigation 119 (2): 305–14. doi:10.1172/JCI35958. PMID 19164858.
- ↑ Chan, et al.; Ogino, S; Fuchs, CS (12 August 209). "Aspirin Use and Survival After Diagnosis of Colorectal Cancer". JAMA 302 (6): 649–658. doi:10.1001/jama.2009.1112. PMID 19671906. PMC 2848289. http://jama.ama-assn.org/cgi/content/short/302/6/649?rss=1.
- ↑ PGxNews.Org (August 2009). "Aspirin use after colorectal cancer diagnosis associated with improved survival". PGxNews.Org. http://www.pgxnews.org/web/pgx-pharmacogenomics-articles-reviews/41-general-literature-review/97-aspirin-use-after-colorectal-cancer-diagnosis-associated-with-improved-survival. Retrieved 2009-08-11.
- ↑ Holmes, M et al (2010). "Aspirin intake and survival after breast cancer". Journal of Clinical Oncology (pre-publication). Available at http://jco.ascopubs.org/cgi/content/abstract/JCO.2009.22.7918v1
- ↑ "Is aspirin a miracle drug?". ABC News, 2010. Available at http://abcnews.go.com/Health/video/aspirin-miracle-drug-9980248
- ↑ Coomer, C (2010). "Aspirin battling breast cancer". Fox News Health Blog, available at http://health.blogs.foxnews.com/2010/02/17/aspirin-battling-breast-cancer/
- ↑ "Women warned aspirin reports may be misleading". National Prescribing Service (2010), available at http://www.nps.org.au/news_and_media/media_releases/repository/Women_warned_aspirin
- ↑ 79.0 79.1 79.2 79.3 79.4 "Aspirin information from Drugs.com". Drugs.com. http://www.drugs.com/aspirin.html. Retrieved 2008-05-08.
- ↑ 80.0 80.1 80.2 "Oral Aspirin information". First DataBank. http://www.personalmd.com/drgdb/3.htm. Retrieved 2008-05-08.
- ↑ Raithel M, Baenkler HW, Naegel A, et al. (September 2005). "Significance of salicylate intolerance in diseases of the lower gastrointestinal tract" (PDF). J. Physiol. Pharmacol. 56 Suppl 5: 89–102. PMID 16247191. http://www.jpp.krakow.pl/journal/archive/0905_s5/pdf/89_0905_s5_article.pdf.
- ↑ Senna GE, Andri G, Dama AR, Mezzelani P, Andri L (1995). "Tolerability of imidazole salycilate in aspirin-sensitive patients". Allergy Proc 16 (5): 251–4. doi:10.2500/108854195778702675. PMID 8566739. http://openurl.ingenta.com/content/nlm?genre=article&issn=1088-5412&volume=16&issue=5&spage=251&aulast=Senna.
- ↑ 83.0 83.1 "PDR Guide to Over the Counter (OTC) Drugs". http://www.mercksource.com/pp/us/cns/cns_hl_pdr.jspzQzpgzEzzSzppdocszSzuszSzcnszSzcontentzSzpdrotczSzotc_fullzSzdrugszSzfgotc036zPzhtm. Retrieved 2008-04-28..
- ↑ Frank B. Livingstone. (1985). G6PD (Glucose-6-Phosphate Dehydrogenase) Deficiency. University of Virginia. ISBN 0195036344. http://www.healthsystem.virginia.edu/uvahealth/adult_blood/glucose.cfm. Retrieved 2008-05-07.
- ↑ Frank B. Livingstone. (1985). G6PD (Glucose-6-Phosphate Dehydrogenase) Deficiency. University of Texas Medical Branch. ISBN 0195036344. http://www.utmbhealthcare.org/Health/Content.asp?PageID=P00091. Retrieved 2008-05-07.
- ↑ "Dengue and Dengue Hemorrhagic Fever: Information for Health Care Practitioners". http://www.cdc.gov/NCIDOD/dvbid/dengue/dengue-hcp.htm. Retrieved 2008-04-28.
- ↑ Dorsch MP, Lee JS, Lynch DR, Dunn SP, Rodgers JE, Schwartz T, Colby E, Montague D, Smyth SS (2007). "Aspirin Resistance in Patients with Stable Coronary Artery Disease with and without a History of Myocardial Infarction". Ann Pharmacother 41 (May): 737. doi:10.1345/aph.1H621. PMID 17456544.
- ↑ Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR (2008). "Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis". BMJ 336 (7637): 195–8. doi:10.1136/bmj.39430.529549.BE. PMID 18202034. PMC 2213873. http://bmj.com/cgi/pmidlookup?view=long&pmid=18202034.
- ↑ Pignatelli P, Di Santo S, Barillà F, Gaudio C, Violi F (2008). "Multiple anti-atherosclerotic treatments impair aspirin compliance: effects on aspirin resistance". J. Thromb. Haemost. 6 (10): 1832–4. doi:10.1111/j.1538-7836.2008.03122.x. PMID 18680540.
- ↑ 90.0 90.1 90.2 Sørensen HT, Mellemkjaer L, Blot WJ, et al. (2000). "Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin". Am. J. Gastroenterol. 95 (9): 2218–24. doi:10.1111/j.1572-0241.2000.02248.x. PMID 11007221. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0002-9270&date=2000&volume=95&issue=9&spage=2218.
- ↑ Delaney JA, Opatrny L, Brophy JM & Suissa S (2007). "Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding". CMAJ 177 (4): 347–51. doi:10.1503/cmaj.070186. PMID 17698822.
- ↑ Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003). "Salicylate induces tinnitus through activation of cochlear NMDA receptors". J. Neurosci. 23 (9): 3944–52. PMID 12736364. http://www.jneurosci.org/cgi/content/full/23/9/3944.
- ↑ 93.0 93.1 Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB (1999). "Reye's syndrome in the United States from 1981 through 1997". N. Engl. J. Med. 340 (18): 1377–82. doi:10.1056/NEJM199905063401801. PMID 10228187. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=10228187&promo=ONFLNS19.
- ↑ NHS Choices: Reye's syndrome. Last reviewed: 16/12/2008 http://www.nhs.uk/conditions/Reyes-syndrome/Pages/Introduction.aspx
- ↑ Berges-Gimeno MP & Stevenson DD (2004). "Nonsteroidal anti-inflammatory drug-induced reactions and desensitization". J Asthma 41 (4): 375–84. doi:10.1081/JAS-120037650. PMID 15281324.
- ↑ Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM. (2009). Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 66(6):714-20. PMID 19364926
- ↑ Gorelick PB. (2009). Cerebral microbleeds: evidence of heightened risk associated with aspirin use. Arch Neurol. 66(6):691-3. PMID 19506128
- ↑ Scher, K.S. (1996). "Unplanned reoperation for bleeding". Am Surg 62 (1): 52–55. PMID 8540646.
- ↑ 99.0 99.1 British National Formulary (45 ed.). British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2003.
- ↑ Aspirin monograph: dosages, etc
- ↑ [1]
- ↑ British National Formulary for Children. British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2006.
- ↑ Gaudreault P, Temple AR, Lovejoy FH Jr. (1982). "The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison". Pediatrics 70 (4): 566–9. PMID 7122154.
- ↑ Marx, John (2006). Rosen's emergency medicine: concepts and clinical practice. Mosby/Elsevier. p. 2242. ISBN 9780323028455.
- ↑ John Robert Vane (1971). "Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs". Nature - New Biology 231 (25): 232–5. PMID 5284360.
- ↑ Vane JR, Botting RM (2003). "The mechanism of action of aspirin" (PDF). Thromb Res 110 (5–6): 255–8. doi:10.1016/S0049-3848(03)00379-7. PMID 14592543. http://www.eao.chups.jussieu.fr/polys/certifopt/saule_coxib/theme/1vane2003.pdf.
- ↑ "Aspirin in Heart Attack and Stroke Prevention". American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier=4456. Retrieved 2008-05-08.
- ↑ Tohgi, H; S Konno, K Tamura, B Kimura and K Kawano (1992). "Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin". Stroke 23 (10): 1400–1403. PMID 1412574.
- ↑ Somasundaram, S. et al.; Sigthorsson, G; Simpson, RJ; Watts, J; Jacob, M; Tavares, IA; Rafi, S; Roseth, A et al. (2000). "Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat". Aliment Pharmacol Ther 14 (5): 639–650. doi:10.1046/j.1365-2036.2000.00723.x. PMID 10792129.
- ↑ Paul-Clark, Mark J.; Cao, Thong van; Moradi-Bidhendi, Niloufar; Cooper, Dianne; Gilroy, Derek W. (2004). "15-epi-lipoxin A4–mediated Induction of Nitric Oxide Explains How Aspirin Inhibits Acute Inflammation". J. Exp. Med. 200 (1): 69–78. doi:10.1084/jem.20040566. PMID 15238606.
- ↑ McCarty, M. F.; Block, K. I. (2006). "Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy". Integr Cancer Ther. 5 (3): 252–268. doi:10.1177/1534735406291499. PMID 16880431.
- ↑ 112.0 112.1 Nye EJ, Hockings GI, Grice JE, Torpy DJ, Walters MM, Crosbie GV, Wagenaar M, Cooper M, Jackson RV (1997). "Aspirin inhibits vasopressin-induced hypothalamic-pituitary-adrenal activity in normal humans". J. Clin. Endocrinol. Metab. 82 (3): 812–7. doi:10.1210/jc.82.3.812. PMID 9062488.
- ↑ Hockings GI, Grice JE, Crosbie GV, Walters MM, Jackson AJ, Jackson RV (1993). "Aspirin increases the human hypothalamic-pituitary-adrenal axis response to naloxone stimulation". J. Clin. Endocrinol. Metab. 77 (2): 404–8. doi:10.1210/jc.77.2.404. PMID 8393884.
- ↑ Ferguson, RK; Boutros, AR (1970-08-17). "Death following self-poisoning with aspirin". Journal of the American Medical Association 213 (7): 1186–8. doi:10.1001/jama.213.7.1186. PMID 5468267.
- ↑ Kaufman, FL; Dubansky, AS (1970-04). "Darvon poisoning with delayed salicylism: a case report". Pediatrics 49 (4): 610–1. PMID 5013423.
- ↑ 116.0 116.1 116.2 Levy, G; Tsuchiya, T (1972-09-31). "Salicylate accumulation kinetics in man". New England Journal of Medicine 287 (9): 430–2. doi:10.1056/NEJM197208312870903. PMID 5044917.
- ↑ Hartwig, Otto H (1983-11-14). "Pharmacokinetic considerations of common analgesics and antipyretics". American Journal of Medicine 75 (5A): 30–7. doi:10.1016/0002-9343(83)90230-9. PMID 6606362.
- ↑ Done, AK (1960-11). "Salicylate intoxication. Significance of measurements of salicylate in blood in cases of acute ingestion". Pediatrics 26: 800–7. PMID 13723722.
- ↑ Chyka PA, Erdman AR, Christianson G, Wax PM, Booze LL, Manoguerra AS, Caravati EM, Nelson LS, Olson KR, Cobaugh DJ, Scharman EJ, Woolf AD, Troutman WG; Americal Association of Poison Control Centers; Healthcare Systems Bureau, Health Resources and Services Administration, Department of Health and Human Services. (2007). "Salicylate poisoning: an evidence-based consensus guideline for out-of-hospital management". Clin Toxicol (Phila) 45 (2): 95–131. doi:10.1080/15563650600907140. PMID 17364628.
- ↑ Prescott LF, Balali-Mood M, Critchley JA, Johnstone AF, Proudfoot AT (1982). "Diuresis or urinary alkalinisation for salicylate poisoning?". Br Med J (Clin Res Ed) 285 (6352): 1383–6. doi:10.1136/bmj.285.6352.1383. PMID 6291695.
- ↑ Dargan PI, Wallace CI, Jones AL. (2002). "An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose". Emerg Med J 19 (3): 206–9. doi:10.1136/emj.19.3.206. PMID 11971828.
- ↑ Katzung (1998), p. 584.
- ↑ Loh HS, Watters K & Wilson CW (1 November 1973). "The Effects of Aspirin on the Metabolic Availability of Ascorbic Acid in Human Beings". J Clin Pharmacol 13 (11): 480–6. PMID 4490672. http://jcp.sagepub.com/cgi/content/abstract/13/11/480.
- ↑ Basu TK (1982). "Vitamin C-aspirin interactions". Int J Vitam Nutr Res Suppl 23: 83–90. PMID 6811490.
- ↑ Ioannides C, Stone AN, Breacker PJ & Basu TK (1982). "Impairment of absorption of ascorbic acid following ingestion of aspirin in guinea pigs". Biochem Pharmacol 31 (24): 4035–8. doi:10.1016/0006-2952(82)90652-9. PMID 6818974.
- ↑ Crosby, Janet Tobiassen (2006). "Veterinary Questions and Answers". About.com. http://vetmedicine.about.com/cs/altvetmedgeneral/a/dogcataspirin.htm. Retrieved 2007-09-05.
- ↑ Cambridge H, Lees P, Hooke RE, Russell CS (1991). "Antithrombotic actions of aspirin in the horse". Equine Vet J 23 (2): 123–7. doi:10.1111/j.2042-3306.1991.tb02736.x. PMID 1904347.
- ↑ Lappin, p. 160
- ↑ Reynolds EF (ed) (1982). Aspirin and similar analgesic and anti-inflammatory agents. Martindale, The Extra Pharmacopoeia 28 Ed, 234-82.
- ↑ Palleros, Daniel R. (2000). Experimental Organic Chemistry. New York: John Wiley & Sons. pp. 494. ISBN 0-471-28250-2.
- ↑ Barrans, Richard. "Aspirin Aging". Newton BBS. http://www.newton.dep.anl.gov/askasci/chem00/chem00314.htm. Retrieved 2008-05-08.
- ↑ Carstensen, J.T.; F Attarchi and XP Hou (1985). "Decomposition of aspirin in the solid state in the presence of limited amounts of moisture". Journal of Pharmaceutical Sciences 77 (4): 318–21. doi:10.1002/jps.2600770407. PMID 4032246.
- ↑ "Acetylsalicylic acid". Jinno Laboratory, School of Materials Science, Toyohashi University of Technology. March 1, 1996. http://chrom.tutms.tut.ac.jp/JINNO/DRUGDATA/07acetylsalicylic_acid.html. Retrieved 2007-09-07.
- ↑ Peddy Vishweshwar, Jennifer A. McMahon, Mark Oliveira, Matthew L. Peterson, and Michael J. Zaworotko (2005). "The Predictably Elusive Form II of Aspirin". J. Am. Chem. Soc. 127 (48): 16802–16803. doi:10.1021/ja056455b. PMID 16316223.
- ↑ Andrew D. Bond, Roland Boese, Gautam R. Desiraju (2007). "On the Polymorphism of Aspirin: Crystalline Aspirin as Intergrowths of Two "Polymorphic" Domains". Angewandte Chemie International Edition 46 (4): 618–622. doi:10.1002/anie.200603373. PMID 17139692.
- ↑ Sigma Aldrich. "Aspirin". http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en&N4=A2093. Retrieved 13 July 2009.
- ↑ British Pharmacopoeia. "Index BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 13 July 2009.
References
- Lappin, Michael R. (2001). Feline Internal Medicine Secrets. Elsevier Health Sciences. ISBN 1560534613.
External links
- NextBio Aspirin Entry
- Colour-enhanced scanning electron micrograph of aspirin crystals
- Interactive 3D-structure of aspirin with detailed x-ray crystal structure data
- The History of Aspirin
- How Aspirin works
- The science behind aspirin
- Take two: Aspirin, New uses and new dangers are still being discovered as aspirin enters its second century. Shauna Roberts, American Chemical Society
- Ling, Greg (2005). "Aspirin". How Products are Made. 1. Thomson Gale. http://www.madehow.com/Volume-1/Aspirin.html.
- U.S. National Library of Medicine: Drug Information Portal - Aspirin
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
|||||